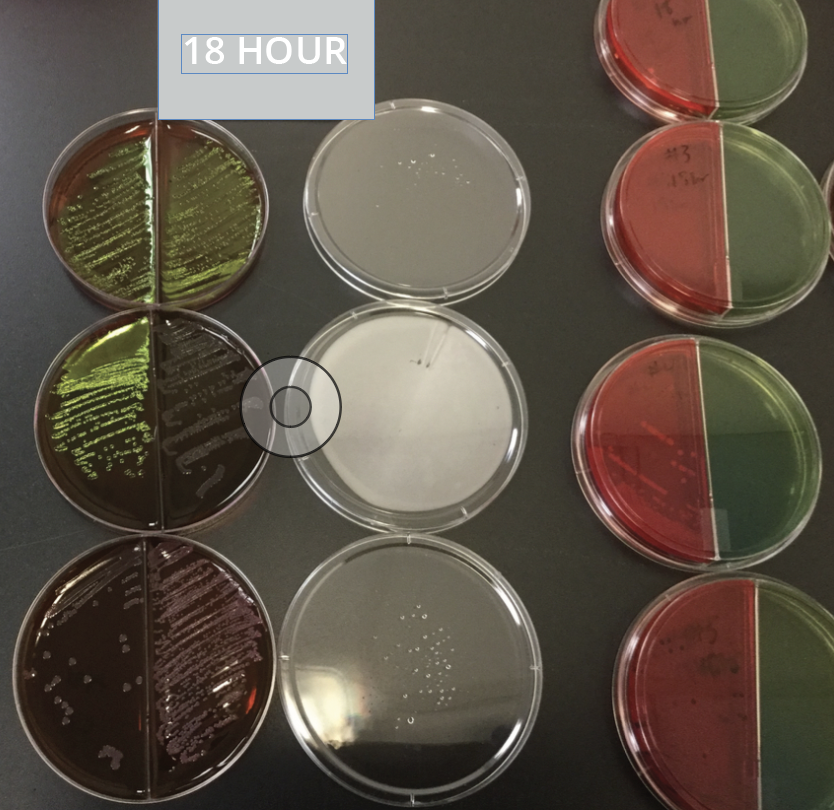
Many analytical labs offer accelerated microbiology testing, but not all of them are using validated methods to achieve the time-saving results. Don’t let your testing fall short of FDA requirements. It’s important to understand the difference between a truly validated testing method, and merely “shortened” method.
The existing USP methods for screening for microbiological pathogens were designed as a multi-step process to detect pathogens in a variety of product types and environments.
Our new Rapid Micro Screening is designed for the nutritional products / supplement industry and has been validated to provide faster test results for both e-coli and salmonella screens.